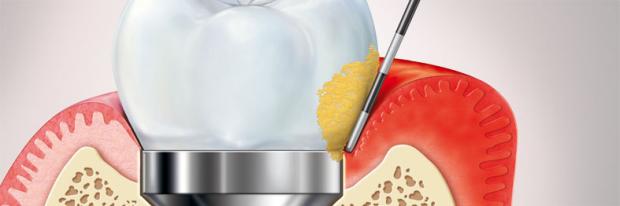
Because peri-implant tissues have a lower capacity to react to the accumulation of oral biofilm compared to periodontal tissues, peri-implant disease is highly prevalent among implant patients. Peri-implant mucositis is the first stage of disease with a prevalence of 28%.
Dental implants are also an opportunity for bacterial colonisation for some species such as Porphyromonas gingivalis, Prevotella intermedia, Actinomyces naeslundii genospecies 2, Fusobacterium nucleatum, Treponema socranskii and Treponema denticola.
Studies show that the ability for biofilm to form on implant surfaces is similar to that observed in natural teeth, because salivary proteins form a film on the implant surface which contains receptors for oral bacterial adhesins.
The implant surface allows for the attachment of salivary proteins, peptides and other substances that form a similar film to that which is formed on natural teeth, containing receptors for the bacterial species adhesins, causing primary colonisation of the implant. These species are similar to the ones that colonise teeth and include members from the Streptococcus, Actinomyces and Veillonella genera.

The formation of pockets is likely to occur around implants, with the ability to hold growing numbers of bacterial species that are known to cause peri-implantitis: P. gingivalis, T. forsythia and A. actinomycetemcomitans, among others.
Peri-implant mucositis is defined as a reversible inflammatory process in the soft tissues surrounding a functioning implant. The continual presence of biofilm on implants induces an inflammatory reaction that may progress to peri-implantitis if not properly treated.
Its expression is similar to gingivitis, and it includes the classic symptoms of inflammation, swelling and redness. Bleeding on probing is a good indicator of peri-implant mucositis.
 initiative
initiative